|
Medtronic has announced a recall of the MiniMed Paradigm insulin pumps Model Series 600 and approximately 60,000 Quick-set infusion sets used with the Medtronic MiniMed Paradigm insulin pumps could be defective and not work properly.
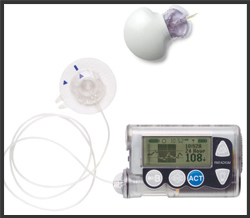 MiniMed Insulin Pumps MiniMed Insulin Pumps
The thin plastic tubes are used with the MiniMed Paradigm Medtronic insulin pump to deliver insulin to diabetes patients. The infusion set is typically replaced every three days. However, thousands of patients may have been sold infusion sets that may not allow the insulin pump to vent air pressure properly, potentially resulting in the device delivering too much or too little insulin.
Over or under delivery of insulin from an insulin pump could have serious and catastrophic consequences for diabetes patients.
Medtronic announced that approximately 60,000 Quick-set infusion sets used with the Medtronic MiniMed Paradigm insulin pumps could be defective and not work properly. Therefore, they recalled an estimated 3 million of the infusion sets with reference numbers MMT-396, MMT-397, MMT-398 and MMT-399 with lot numbers starting with an “8”.
Medtronic Class I Recall: Model 600 Series
Medtronic has announced the recall of the MiniMed Paradigm insulin pumps Model 630 (MMT-1715) and Model 670G(MMT-1780). The FDA has issued a notice and has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. Read the FDA Recall Notice - MiniMed 600 Series Insulin Pump.
Medtronic Class I Recall of Faulty Insulin Pumps 2013
The recall of the Medtronic Insulin Pump is the result of a mechanical issue involving a part used in the Paradigm Insulin Pump. The mechanical issue could lead to the delivery of too much or too little insulin. The FDA has upgraded this mechanical issue to a Class I recall which is the FDA’s most serious label. On June 7th 2013, Medtronic issued an “urgent medical device safety notification” that stands as a warning that its Paradigm insulin pump could fail and give too much or too little insulin or that other fluids could touch the inside of the system’s tubing connectors resulting in the patient becoming very sick from hypoglycemia or “hyperglycemia”. Furthermore, the failure could block the vents that allow the pump to work correctly. Regulators noted, Medtronic produced the affected insulin pump model from October 2001 through June 2013 and distributed the defective devices from December 2001 through June 2013. The FDA is warning patients to not insert the affected infusion set and be aware of issues where the insulin continues to drip from the tip of the infusion set cannula after priming has been completed.
Insulin Overdose Symptoms
Diabetes patients who begin to develop any of the following signs or symptoms should seek medical attention immediately as they may be experiencing an insulin overdose:
- Severe headache
- Increased/rapid heartbeat
- Nausea
- Tremors, anxiety
- Uncontrolled sweating
- Hypoglycemia
Hypoglycemia
One of the most dangerous risks associated with insulin overdose is hypoglycemia, a condition in which a person’s blood sugar (glucose) is too low. Symptoms include: cold sweats, confusion, double vision, convulsions, fatigue and general discomfort. Severe hypoglycemia can result in seizures, coma and death.
If you or a loved one has suffered complications from MiniMed Insulin Pumps, please contact the Steinberg Law Firm P.C. at 888-529-4688 or email andrewsteinberg@lawyer.com.
Consult A Lawyer on Legal Issues
We encourage you to contact us at 888-529-4688 to discuss your MiniMed Insulin Pump case with an experienced personal injury and wrongful death attorney.
| » Fill out this form if you need additional information or want to discuss a potential claim with an attorney. All inquiries are kept strictly confidential. Please e-mail Andrew E. Steinberg* at: andrewsteinberg@lawyer.com for a free, confidential consultation, or call us at 888-529-4688. |
| » If the inquiry is accepted for further review, you will receive a prompt response (usually the same day or by the next business day). |
Consult A Doctor On Medical Issue
Please consult your doctor, not your lawyer, on matters relating to your health. It could be dangerous to stop taking medicines, especially abruptly. Patients should talk to their physicians to decide whether the benefits and risks of using MiniMed Insulin Pumps make it the right choice for them.
|